Airo® TruCT®
Great procedures start with great imaging.When you see more, you can do more.
Features and benefits
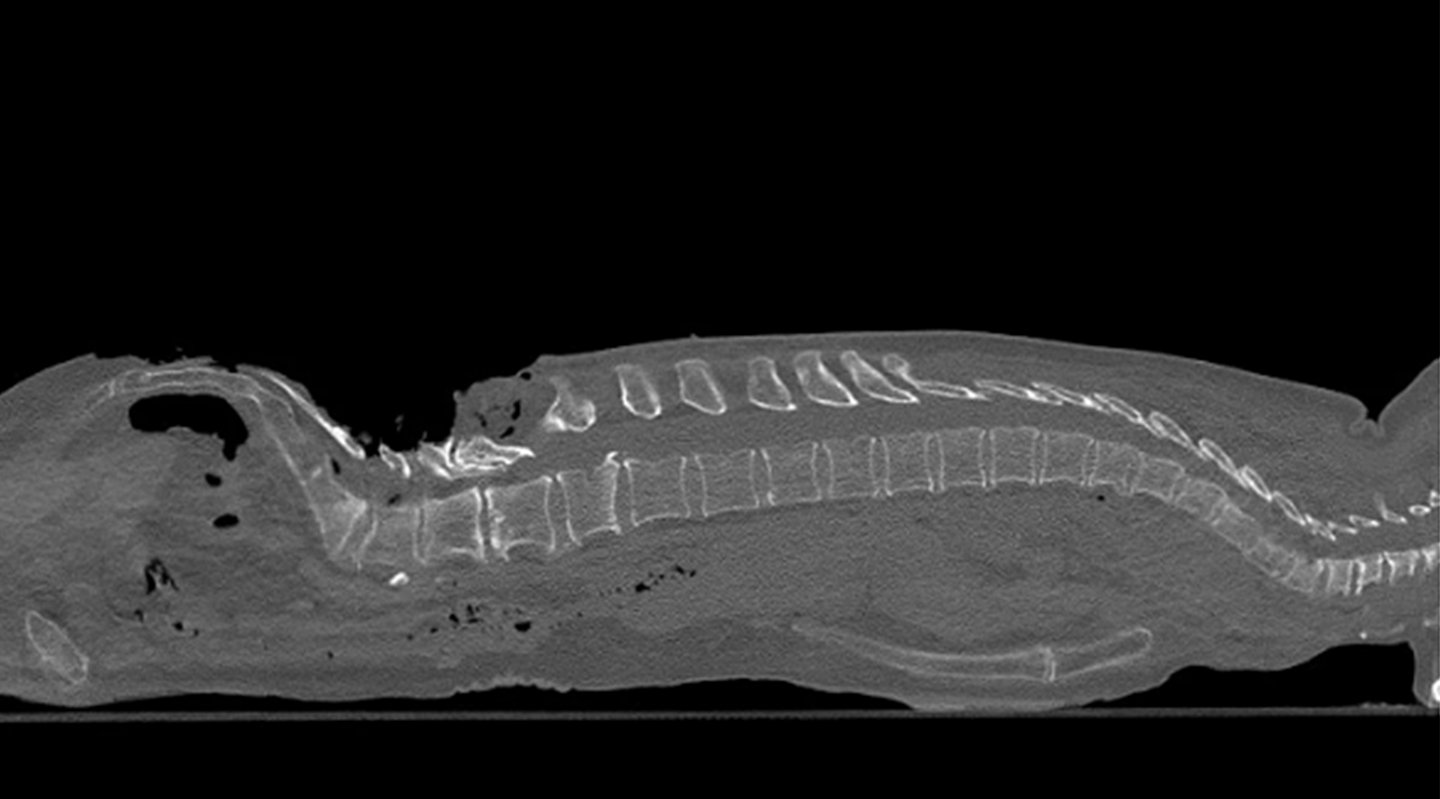
- Scan one full meter of the spine (up to 51.2 cm x 100 cm scan volume)in a single scan in as little as 43 seconds1
- The Airo TruCT considers your patient’s unique anatomy. It is designed to deliver customized radiation and potentially minimize radiation exposure2
- Expand your capabilities with a 107 cm diameter bore for patient scans and positioning
- Support clinical decision-making with a 32-slice detector array providing details anatomical imagine for bony and soft tissue
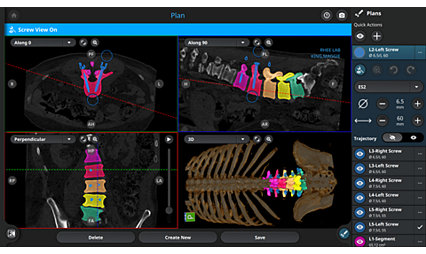
- High-resolution images uploaded intra-operatively to the Q GuidanceSystem with Spine Guidance Software
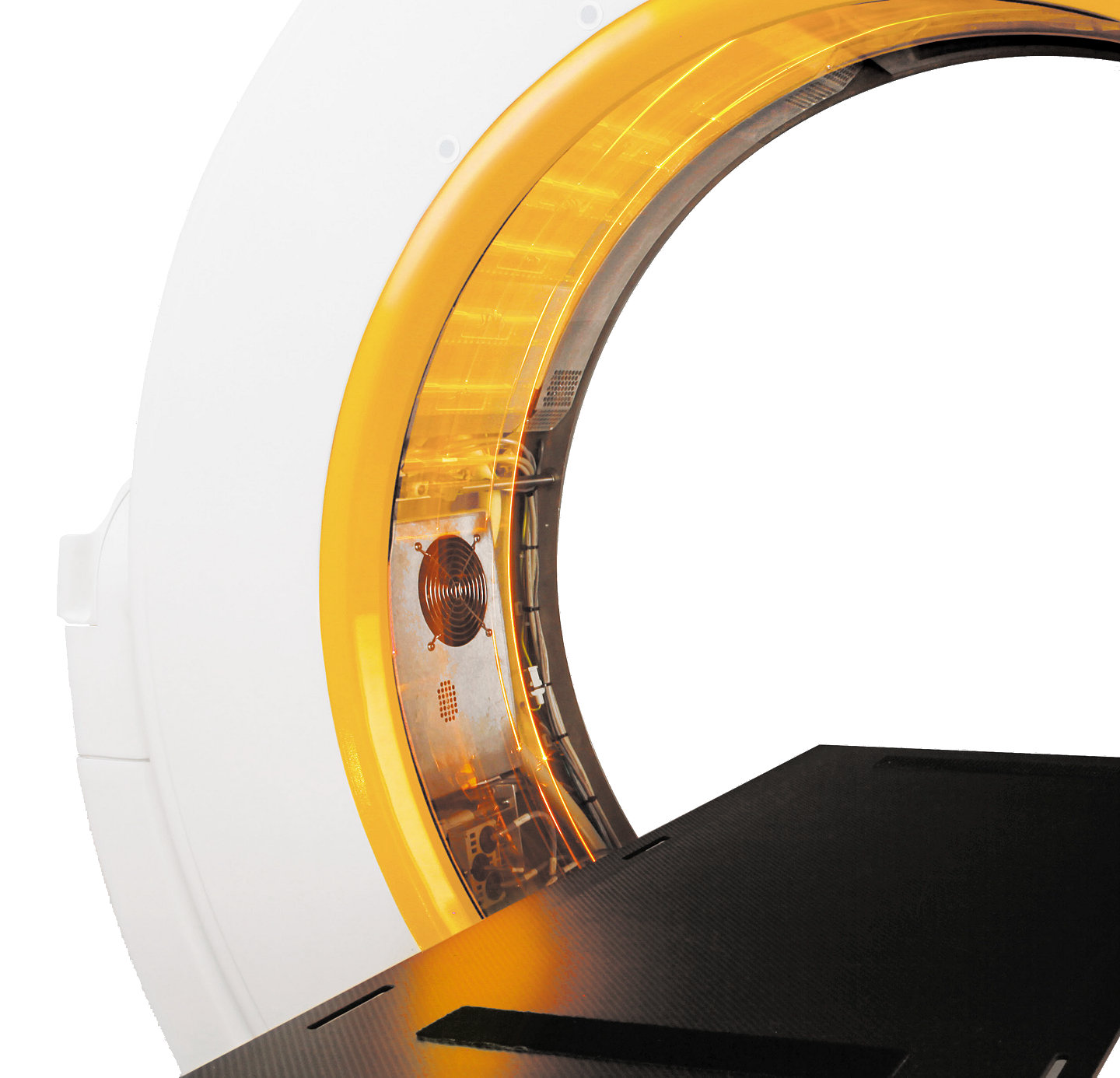
Image library
Great procedures start with great imaging. View the image library to see innovative fan beam technology imaging.
Informational video

Airo TruCT supports the demanding requirements of intra-operative imaging in spine surgery.
Watch the video to learn more.
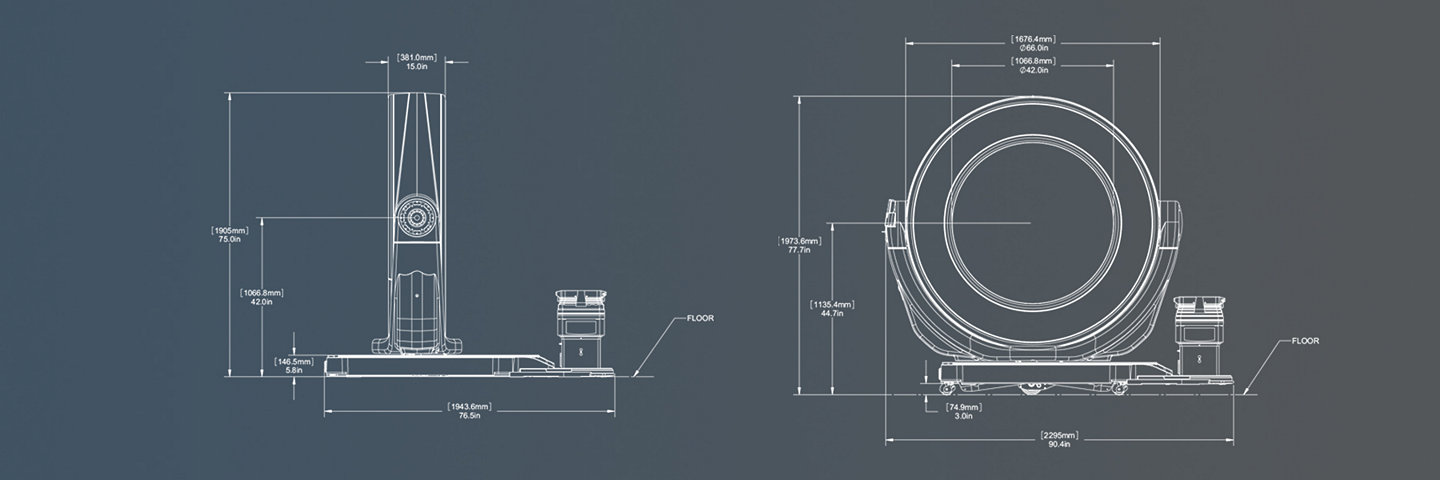
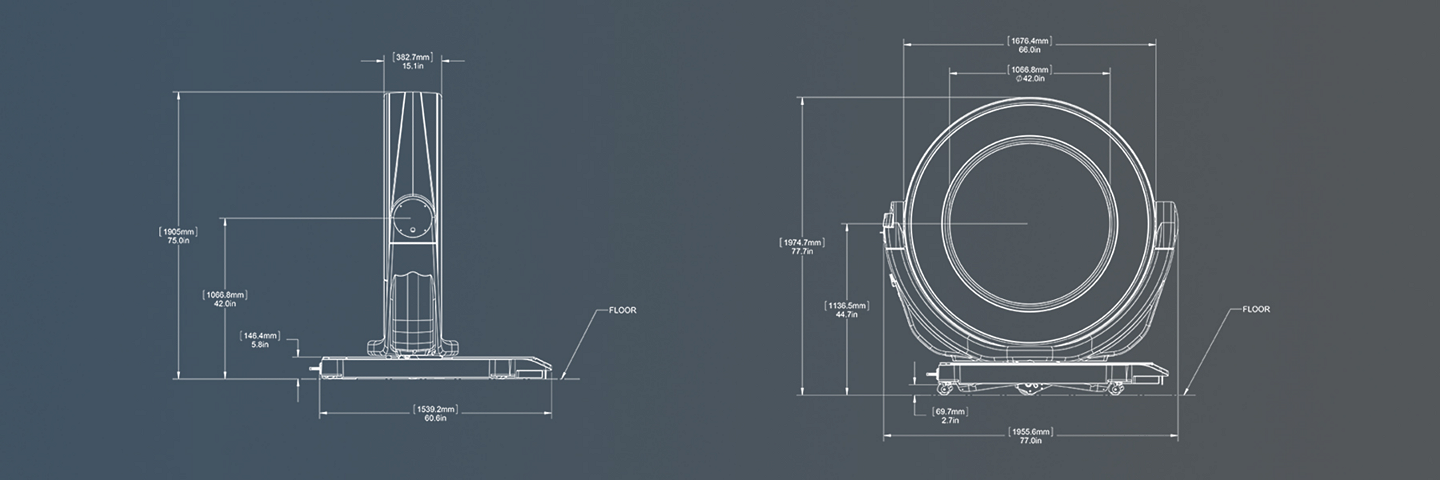
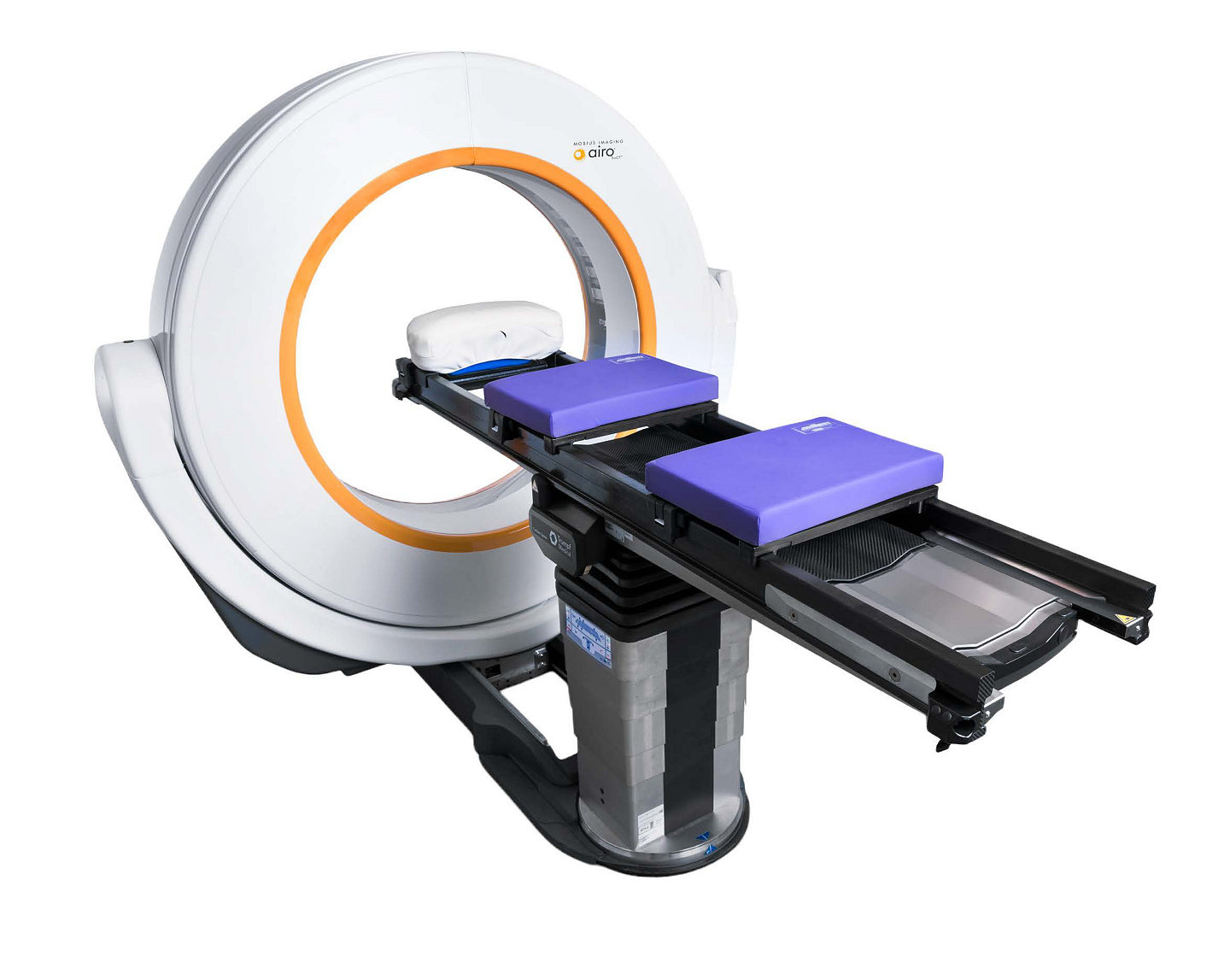
Airo TruCT | system dimensions
Scan mode
In scan mode, the base is lowered onto the ground by retracting the casters in the support base. The Ring is rotated 90 degrees perpendicular to the base. In scan mode, all functionality other than transport is available to the user, such as patient loading, system positioning, image acquisition, and image processing. The Pendant provides a user interface which enables viewing and manipulation of stored images from the patient database. Images can be archived to a USB digital storage media or pushed to a PACS or a navigation system through DICOM protocol.
Transport mode
In transport mode, the Ring is aligned with the base, and the entire system is powered from a battery pack located on the Ring. In this mode, Airo can be driven and positioned by the control Pendant attached to one side of the Gimbal. Moving the Airo typically requires only a single person even when taking the system up or down a ramp. However, a second person may act as a spotter in busy or crowded hallways. Once the Airo has been moved to the desired location (for storage or scanning), it is then plugged into a standard power outlet for recharging.
Airo TruCT | system specifications
Bore (Aperture): 107 cm
Gantry width: 38 cm
Image field of view: 51.2 cm
Helical scanning capabilities
Rotation time: 1.92 sec
Scan range: 1 meter
Scan time: 43 sec for 1 meter scan range1
Helical scan pitch: 1.415
Slice thickness: 32 x 1.0 mm
Image reconstruction time: 24 images per sec
X-ray generation
X-ray tube voltage: 80, 100, 120 kV
X-ray tube current: 5 - 250 mA
Focal spot size: 1.0 x 1.0 mm
Image quality
Reconstruction matrix: 512 x 512
Low contrast detectability: 3 mm at 0.5%
X-ray detection
Detection system: Solid-State Array
Main detectors: 32 rows
Size and mobility
Weight of 816 kg (1800 lbs) + optional column 159 kg (350 lbs)
Airo contains a powered-drive system that can transport at a fixed rate of approximately 1 mph in the forward direction and 0.5 mph in the reverse direction.
Related products
Spine Guidance Software
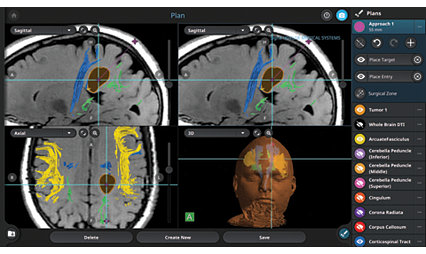
Learn moreCranial Guidance Software
Learn moreReferences
- MI-19-0030 | Airo Design Verification Procedure
- Testing reference MI-48-0423 | Using the optional X-ray tube current modulation feature. As the scan translates, radiation is adjusted to the patient’s specific anatomy based on the scout scan. This will come onscreen as this section is read. This feature is only available on Airo Software version 1.2 and higher.)
TruCT-WB-4_34832